Long Covid
Setting Expectations
This is not a normal website. We have specifically designed this content to first educate you on everything Long Covid and then to assist you in having that first conversation with your patients.
Our strategy will start by identifying the issue of Spike Persistence and its role in Long COVID, along with the associated symptoms. We will evaluate the magnitude of this problem to grasp its full impact. Subsequently, we will catalog the potential causes, offering a detailed examination of the factors that may contribute to Spike Persistence and Long COVID. Our investigation will probe into the underlying mechanisms, exploring the biological processes and interactions at play. We will pinpoint the regions or aspects where the issue is most pronounced, underscoring the areas in need of urgent intervention.
Spoiler Alert: It’s the Spike Protein that is the really bad actor and it needs to be dealt with NOW!
Identifying The Problem
What is Long Covid & Persistent Spike Protein?
Persistent Spike Protein that has not been removed by the immune system of the body is the contributing factor for Long Covid. Long Covid is a persistent Spike illness because the immune system is too weak to clean it out. This can result in a variety of clinical presentations. Ongoing research and studies by our team and many others around the world continue to discover new and alarming ways in which this Spike Protein invades and takes up residence in a plethora of cell types.
These cell types include astrocytes, endothelial cells, and neurons, contributing to the wide range of symptoms and complications observed in Covid-19 patients.
As of now, we can say that Long Covid is a complex condition characterized by diverse symptoms affecting the heart, lungs, blood, and nervous system, often without significant findings in patient-reported symptoms. The similarity of Long Covid to conditions such as myalgic encephalomyelitis/chronic fatigue syndrome and postural orthostatic tachycardia syndrome (POTS), along with other post-viral syndromes, typically leads to its identification through a process of elimination.As you can imagine, this makes diagnosis and treatment a real challenge.
What are the Symptoms of Long Covid?
Long Covid symptoms are wide and varied and differ dramatically from one person to the next. Indeed, even the term Long Covid itself has a variety of similar naming conventions. Other terms that have been used to describe Long Covid include “Post-Covid Syndrome” (United Kingdom), “Post Covid-19 Conditions” (World Health Organization) or “Post-Acute Sequelae of Covid-19” (abbreviated to PASC) (United States). The World Health Organization (WHO) regional office for Europe and the National Institute for Health Research (NIHR) have used the term Long Covid. “Post Covid-19 Conditions” is the term now used by the World Health Organization (WHO) and the United States Centers for Disease Control and Prevention (CDC).
To make things even more complicated, there is a common set of patient symptoms during infection, post infection and post vaccination. As such, differentiating symptoms is challenging.
The diagram below is a good illustration of potential Post & Long Covid symptoms.
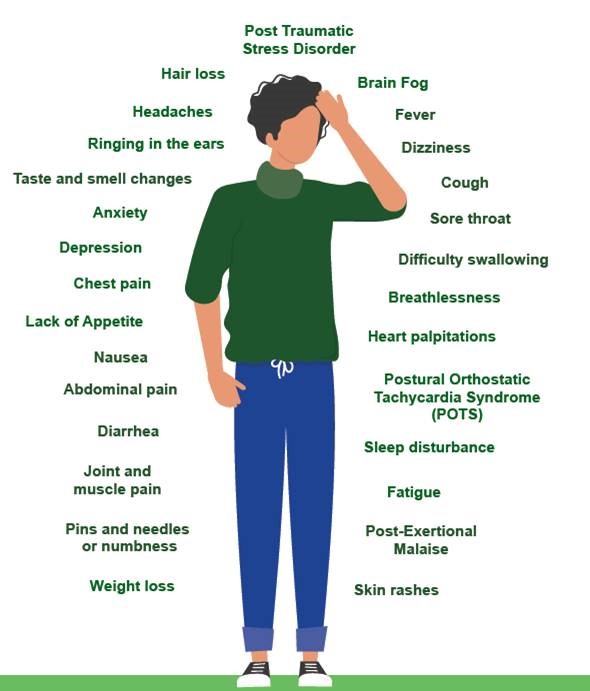
How many people are presently affected by Long Covid?
Here are some numbers on Long Covid that show a large population of people suffering today and likely well into the future.
The World Health Organization (WHO) states that Long Covid can affect anyone who has been exposed to SARS-CoV-2, regardless of the severity of their original symptoms. Studies indicate that around 10–20% of people infected by SARS-CoV-2 may develop symptoms that can be diagnosed as Long Covid.
The Office for National Statistics (ONS) in the UK reports that Long Covid symptoms adversely affected the day-to-day activities of 77% of those with self-reported Long Covid, with 19% reporting that their ability to undertake their day-to-day activities had been “limited a lot”.
Harvard Medical School’s study found that Long Covid “long haulers” in the U.S. were more likely to be older and female, with more chronic conditions than those who did not have diagnosed Long Covid.
The NHS England states that Long Covid can affect people of all ages, with the most common symptoms including extreme tiredness (fatigue), shortness of breath, and muscle aches].
The Mayo Clinic notes that research suggests that between one month and one year after having Covid-19, 1 in 5 people ages 18 to 64, and 1 in 4 people age 65 and older, may have at least one medical condition that might be due to Covid-19.
The Centers for Disease Control and Prevention (CDC) provides various estimates regarding the number of people affected by Long COVID in the United States:
- According to a report published by the CDC’s National Center for Health Statistics, in 2022, 6.9% of adults reported ever having Long COVID, while 3.4% said they currently had the condition at the time of the interview. This translates to about 18 million adults who have experienced Long COVID at some point and approximately 8.8 million who are currently experiencing it, based on 2022 U.S. Census estimates.
- The CDC also estimated that as of the end of 2022, about 7% of American adults had experienced Long COVID at some point, with the condition affecting their day-to-day activities. Among those, 19% reported that their ability to undertake day-to-day activities had been “limited a lot”.
- Another CDC survey found that as of January 16, 2023, 15% of all adults in the US reported having had Long COVID symptoms at some point, and 6% reported current symptoms..
- The CDC’s Household Pulse Survey from June 2022 to December 2022 reported that 6.9% of weighted respondents were currently experiencing Long COVID symptoms that reduced their ability to carry out day-to-day activities.
- The CDC’s National Health Interview Survey results released in 2022 showed that roughly 18 million Americans said they have ever had Long COVID, and 8.8 million said they currently have the condition.
These figures from the CDC indicate that Long COVID is a significant public health concern, affecting millions of Americans with varying degrees of impact on their daily lives.
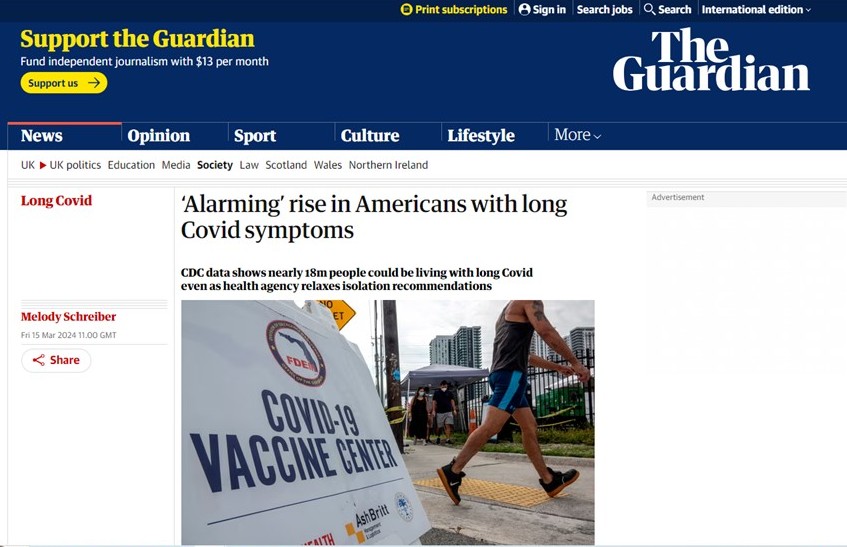
In summary, Long Covid is affecting millions of people in the United States and around the world. The prevalence of Long Covid is even being reported in mainstream media:
https://www.theguardian.com/world/2024/mar/15/long-Covid-symptoms-cdc
Understanding the Spike Protein
What is the Spike Protein?
The spike Protein is a structure on the surface of the SARS-CoV-2 virus, which causes COVID-19. It is a key component that allows the virus to enter and infect human cells. The spike Protein binds to the angiotensin-converting enzyme 2 (ACE2) receptor on the surface of a cell, facilitating the virus’s entry. The persistence of the spike Protein in the body has been associated with Long COVID, with individuals experiencing ongoing symptoms after the acute phase of the infection.
The Spike Protein enters various cell types
SARS-CoV-2 spike Protein can enter into various cell types, including astrocytes, endothelial cells, and neurons, as demonstrated by several studies.
Astrocytes, which are a type of glial cell in the brain, have been shown to be susceptible to SARS-CoV-2 infection. Research has found that on average, SARS-CoV-2 spike Protein was detected in 37% of the cells in brain tissue samples from COVID-19 patients, with the majority of these spike-positive cells being astrocytes (65.93%). This suggests that approximately 25% of all cells in the brain samples analyzed were infected astrocytes. The study also confirmed the presence of viral genetic material and spike Protein in infected astrocytes, indicating viral replication within these cells. Furthermore, SARS-CoV-2 infection was observed to reduce human astrocyte viability by 25% after 48 hours post-infection, a condition that persisted after 72 hours[1].
Study Link: https://www.medrxiv.org/content/10.1101/2020.10.09.20207464v4.full.pdf
Endothelial cells, which line the interior surface of blood vessels, have also been found to be infected by SARS-CoV-2 in vivo and in vitro. The research highlighted the presence of SARS-CoV-2 spike RNA within endothelial cells and demonstrated that the virus can infect mature vascular endothelial cells, potentially contributing to cardiovascular complications in COVID-19, including multiple organ failure. The study also discussed the role of alternative receptors or host factors in the entry of SARS-CoV-2 into host cells, suggesting that the mechanism by which SARS-CoV-2 infects endothelial cells remains to be fully understood.
Study Link: https://www.frontiersin.org/articles/10.3389/fcimb.2021.701278/full
Neurons, the principal cells of the brain responsible for receiving sensory input from the external world, processing and transmitting information throughout the body, have also been reported to be susceptible to SARS-CoV-2 infection. Studies have indicated that SARS-CoV-2 can infect neurons and replicate within them. This is supported by observations of viral particles budding from the endoplasmic reticulum of neurons, indicating active viral replication.
Study Link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8062881/
Study Link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8994061/
In summary, SARS-CoV-2 spike Protein can enter and persist in various cell types, including astrocytes, endothelial cells, and neurons, contributing to the wide range of symptoms and complications observed in COVID-19 patients.
The Spike Protein is persisting in the body
Here are studies showing the persistence of the spike Protein.
- SARS-CoV-2 Spike Protein Accumulates in the Skull and Brain: This study found SARS-CoV-2 spike Protein in the skull marrow, meninges, and parenchyma in mouse models and human postmortem samples. Injecting spike Protein into skull marrow niches of healthy mice triggered proteome changes and cell death in the brain. The findings suggest that spike Protein in the skull-meninges-brain axis may be a molecular mechanism or therapeutic target for addressing the lingering effects of SARS-CoV-2 infection.
Media Link : https://www.medpagetoday.com/neurology/longcovid/104037
Study Link: https://www.biorxiv.org/content/10.1101/2023.04.04.535604v1 - Long COVID: New evidence for ‘viral reservoirs’, biomarker in body: Researchers from Harvard Medical School and the Ragon Institute found that the spike Protein was present in the blood of a majority of long COVID patients up to 12 months after their initial diagnosis. The study indicates that the presence of spike Protein in the blood could indicate a persistent viral reservoir in the body.
Media Link: https://www.medicalnewstoday.com/articles/long-covid-viral-reservoir-of-spike-Protein-may-explain-long-term-symptoms|
Study Link: https://www.medrxiv.org/content/10.1101/2022.06.14.22276401v1.full.pdf - Circulating Spike Protein Detected in Post–COVID-19 mRNA Vaccine Myocarditis: This study discovered that individuals who developed post vaccine myocarditis exhibited elevated levels of free spike Protein in circulation, suggesting a unique response to the spike Protein in these cases.
StudyLink: https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.122.061025 - Could SARS-CoV-2 Spike Protein Be Responsible for Long-COVID Syndrome?: This paper discusses the potential role of the spike Protein in causing long-COVID syndrome. It highlights findings that the spike Protein could damage the endothelium in an animal model and disrupt the blood-brain barrier, leading to perivascular inflammation and potentially contributing to long-COVID symptoms.
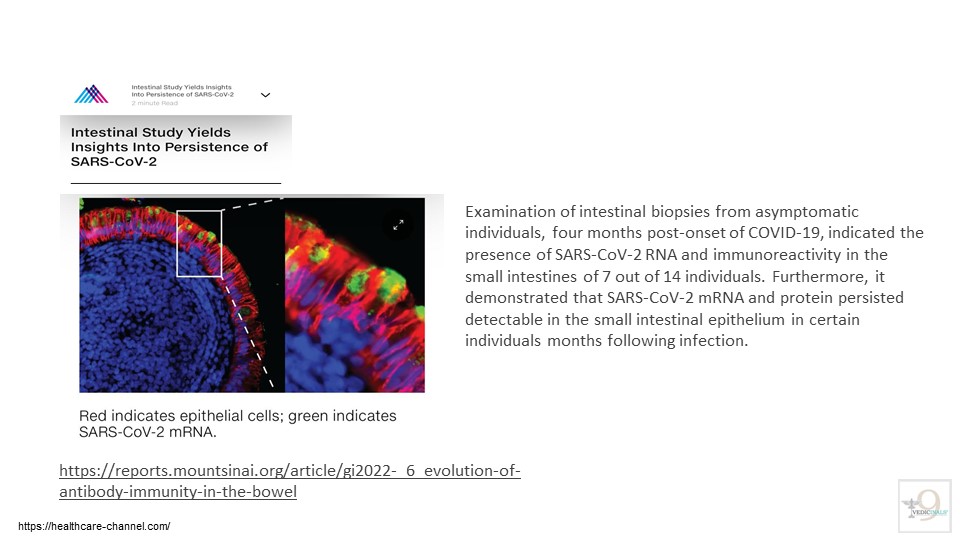
- Researchers from Mount Sinai and Rockefeller University highlighted the persistence of the virus in the gut, with SARS-CoV-2 RNA and Protein detectable in the small intestines of some individuals months after infection. This ongoing viral presence might enhance the specificity of the antibody response but could also contribute to “long COVID,” a condition affecting up to a third of COVID-19 patients with diverse and prolonged symptoms. The Mount Sinai-Rockefeller University research has led to further studies exploring the relationship between viral persistence and long-COVID symptoms.
Study Link: https://reports.mountsinai.org/article/gi2022-_6_evolution-of-antibody-immunity-in-the-bowel
These studies confirm the persistence of the spike Protein in the body and its potential implications for long COVID and other health conditions.
The Consequences of Persistent Spike Protein
The persistence of the SARS-CoV-2 spike Protein in the body is implicated in the pathogenesis of Long COVID through several mechanisms. Here’s a detailed explanation of just a few mechanisms of action identified to date.
- Viral Reservoirs and Persistent Infection: The presence of the SARS-CoV-2 spike Protein in the body up to 12 months after the initial infection suggests the existence of viral reservoirs, particularly in organs such as the gut. These reservoirs allow the virus to evade the immune system, leading to a persistent infection that can cause ongoing symptoms associated with long COVID. The gut, for example, has been identified as a possible reservoir, with individuals showing detectable viral RNA in their stools also reporting ongoing gastrointestinal symptoms.
Media Link: https://www.medicalnewstoday.com/articles/long-covid-viral-reservoir-of-spike-Protein-may-explain-long-term-symptoms
Study Link: https://www.biorxiv.org/content/10.1101/2023.04.04.535604v1 - Continued Inflammation & Thrombosis: The spike Protein of the SARS-CoV-2 virus is known to trigger immune responses. In some cases, even after the acute phase of the infection has resolved, remnants of the spike Protein or viral particles may persist in the body. This persistent presence can lead to chronic inflammation, which is associated with many of the symptoms seen in long COVID, such as fatigue, brain fog, and muscle pain.
- Immune System Activation: The persistence of the spike Protein can continuously activate the immune system. This ongoing immune response can lead to inflammation and damage to multiple organs, contributing to the wide range of symptoms observed in long COVID. The presence of virus-infected cells at low levels could also serve as a “trigger” for this continued immune activation.
Media Link: https://www.scientificamerican.com/article/people-with-long-covid-may-still-have-spike-Proteins-in-their-blood1/ - Neurological effects: The accumulation of SARS-CoV-2 spike Protein in the skull-meninges-brain axis can contribute to changes in the brain, suggesting a possible mechanism for the neurological effects of SARS-CoV-2 infection. The presence of spike Protein in the brain and meninges from the same patients, even in the absence of viral load, suggests either a specific uptake mechanism to the brain or a longer half-life of spike Protein compared to other Proteins. The persistence of spike Protein in the skull marrow, brain meninges, and brain parenchyma may contribute to long-term neurological symptoms in COVID-19 patients, as shown by the presence of spike Protein in the skull of deceased patients long after their infection.
Study Link: https://www.biorxiv.org/content/10.1101/2023.04.04.535604v1.full.pdf
Study Link: https://www.biorxiv.org/content/10.1101/2023.04.04.535604v1.full - Autoimmunity: The spike Protein shares antigenic epitopes with human molecular chaperones, which can lead to autoimmunity. This autoimmune response can result in the release of inflammatory cytokines, further contributing to the symptoms of long COVID. Prolonged exposure to the spike Protein may trigger autoimmune reactions in susceptible individuals. The immune system, in its efforts to clear the virus, may mistakenly target healthy tissues and organs, leading to a range of symptoms.
Study Link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8757925/ - Altered neuronal phenotypes: SARS-CoV-2 spike Protein has been found to reduce burst activities in neurons, specifically in neurons exposed during early development, which may have implications for neurological development and function.
Study Link: https://www.nature.com/articles/s41401-022-00998-0 - Complement and coagulation pathway dysregulation: The spike Protein has been associated with neutrophil-related pathways and dysregulation of the Proteins involved in the PI3K-AKT pathway, complement, and coagulation pathways, which may contribute to the pathogenesis of COVID-19.
Study Link: https://www.biorxiv.org/content/10.1101/2023.04.04.535604v1 - Endothelial Damage: The spike Protein can damage the endothelium (the inner lining of blood vessels). This damage can disrupt normal blood flow and contribute to the vascular issues seen in long COVID patients.
Study Link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8757925/
The mechanism by which the SARS-CoV-2 spike Protein affects endothelial function is through various pathways, including the disruption of junctional Proteins that maintain endothelial barrier integrity, activation of the complement system, and induction of inflammation.
- Disruption of junctional Proteins: SARS-CoV-2 spike Protein can induce degradation of junctional Proteins that maintain endothelial barrier integrity, such as VE-Cadherin, PECAM-1, JAM-A, and Connexin-43. This disruption can lead to impaired endothelial barrier function and wider cardiovascular complications.
Study Link: https://www.frontiersin.org/articles/10.3389/fcvm.2021.687783/full - Complement activation: The spike Protein can directly activate the alternative complement pathway, leading to complement Protein deposition and activation of the coagulation cascade, which may contribute to thrombosis and endothelial dysfunction.
Study Link: https://ashpublications.org/blood/article/136/18/2080/463611/Direct-activation-of-the-alternative-complement - Inflammation: SARS-CoV-2 spike Protein can bind to ACE2, the primary receptor used by the virus for cellular entry, and induce inflammation in endothelial cells. This process is independent of viral replication and can lead to increased production of IL-6, MCP-1, ICAM-1, and PAI-1, and NFkB activation via ACE2 in endothelial cells.
Study Link: https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2022.827146/full - Endothelial injury: The spike Protein can increase microparticle formation, a functional marker of endothelial injury, in human endothelial cells.
Study Link: https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2022.827146/full
These findings suggest that SARS-CoV-2 spike Protein can directly affect endothelial function by inducing inflammation, endothelial injury, and complement activation, which may contribute to the cardiovascular complications observed in COVID-19 patients.
Overall, there is evidence now showing that persistent Spike Protein in the body is causing a variety of chronic conditions.
